Overview
For the first time 5 HMOs (Human Milk Oligosaccharides) that combine all 3 major HMO categories will be available in an infant formula. The first ever clinical study with a unique 5HMO blend has shown that the formula is safe and supports age-appropriate growth similar to breastfed infants.1
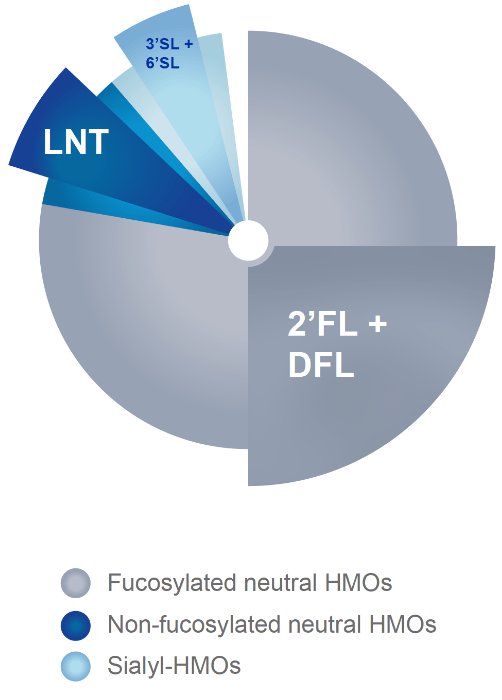
The 5 HMOs in SMA® ADVANCED FORMULAS
- Are among the most abundant, quantitatively accounting for more than 40% of the 20 HMOs generally measured in breastmilk.2-6
- Together they represent the three HMO families.7
- Are uniquely combined in proportions based on HMO ratios in breast milk.8
- Are structurally identical to HMOs in breast milk.9-18
- Are generally recognised as safe by the US FDA (GRAS) and evaluated as safe by EFSA.9-18